appears in the following:
High Prices For Orphan Drugs Strain Families And Insurers
Tuesday, January 17, 2017
Three decades ago, Congress set up a system to encourage drug companies to develop treatments for rare diseases. The law has worked, but at a high cost.
Drugs For Rare Diseases Have Become Uncommonly Rich Monopolies
Tuesday, January 17, 2017
Drugmakers have brought almost 450 orphan drugs to market and collected rich incentives by doing so. But nearly a third of the medicines aren't new or were repurposed many times for financial gain.
Winners And Losers If 21st Century Cures Bill Becomes Law
Friday, December 02, 2016
Sweeping legislation that would reshape how the Food and Drug Administration reviews new products and bolster National Institutes of Health funding is moving closer to becoming law. What's at stake?
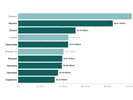
Legislation That Would Shape FDA And NIH Triggers Lobbying Frenzy
Friday, November 25, 2016
More than 1,455 lobbyists weighed in on the 21st Century Cures Act in this congressional cycle. Only one health bill since 2011 has garnered more attention.
A Look At How The Revolving Door Spins From FDA To Industry
Wednesday, September 28, 2016
About 27 percent of Food and Drug Administration reviewers who approved hematology-oncology drugs from 2001 through 2010 left to work for the industry they previously regulated, an analysis found.
FDA Fees On Industry Haven't Fixed Delays In Generic Drug Approvals
Thursday, September 01, 2016
Four years after user fees were imposed to speed up the review of generic drug applications by the Food and Drug Administration, more than 4,000 generics remain in limbo.